The research and development activities at HeMoLab are grouped within the lines of research briefly described below. Regarding modeling techniques, most of these activities share strong variational foundations. Numerics is handled through finite element methods, finite volume methods, finite difference methods and optimization algorithms based on sensitivity analysis, among others.
| 1D Cardiovascular Simulation | |
|
expand
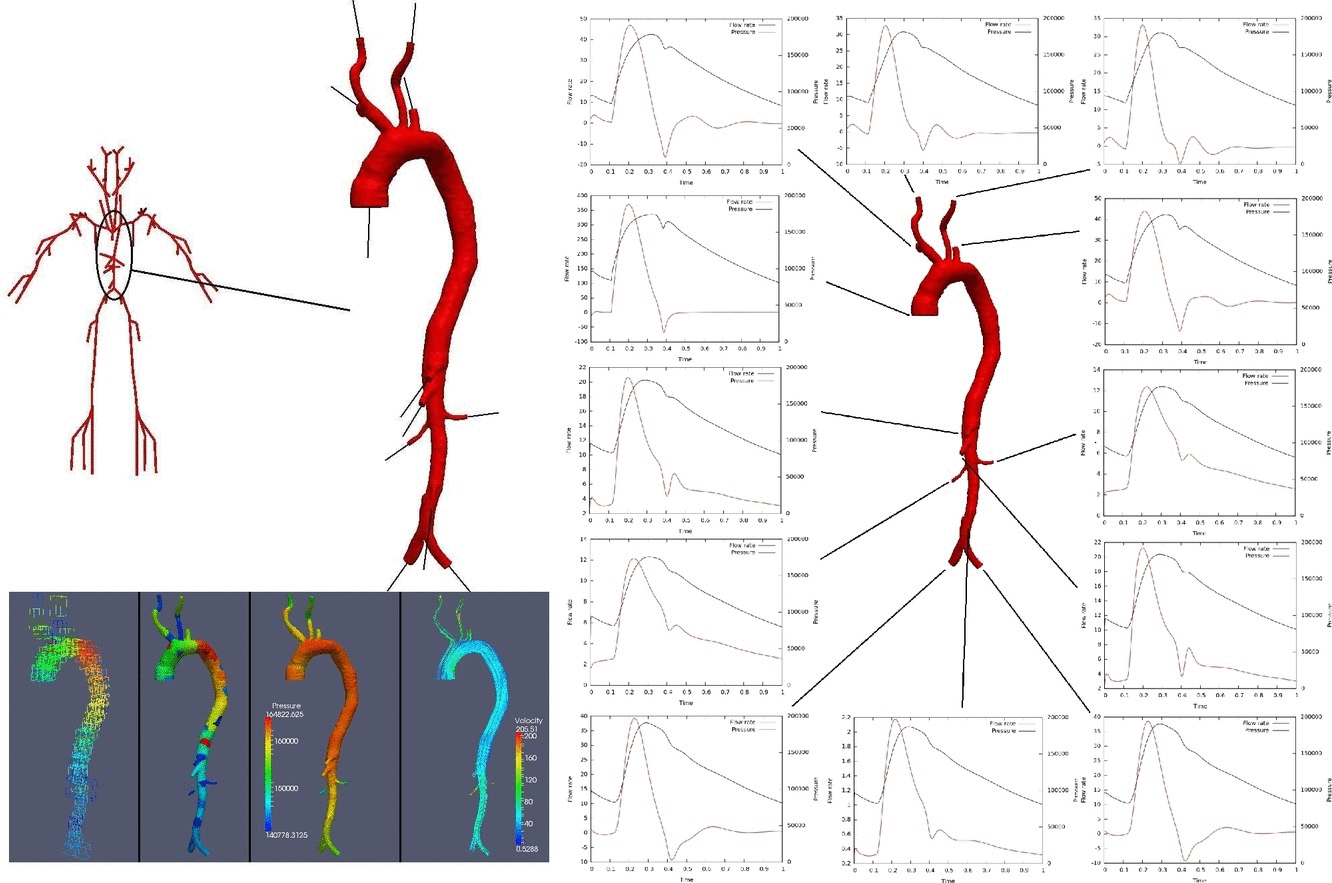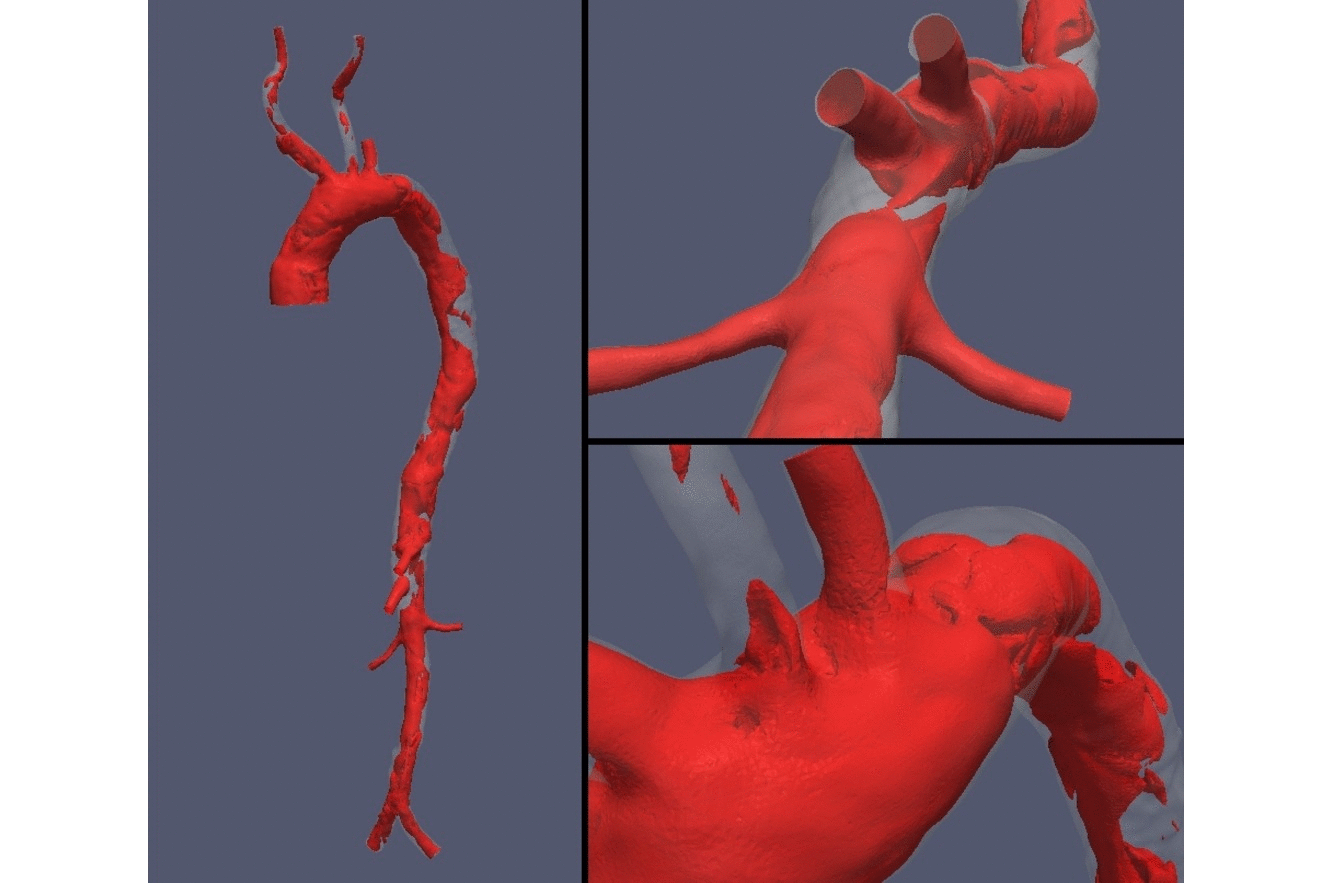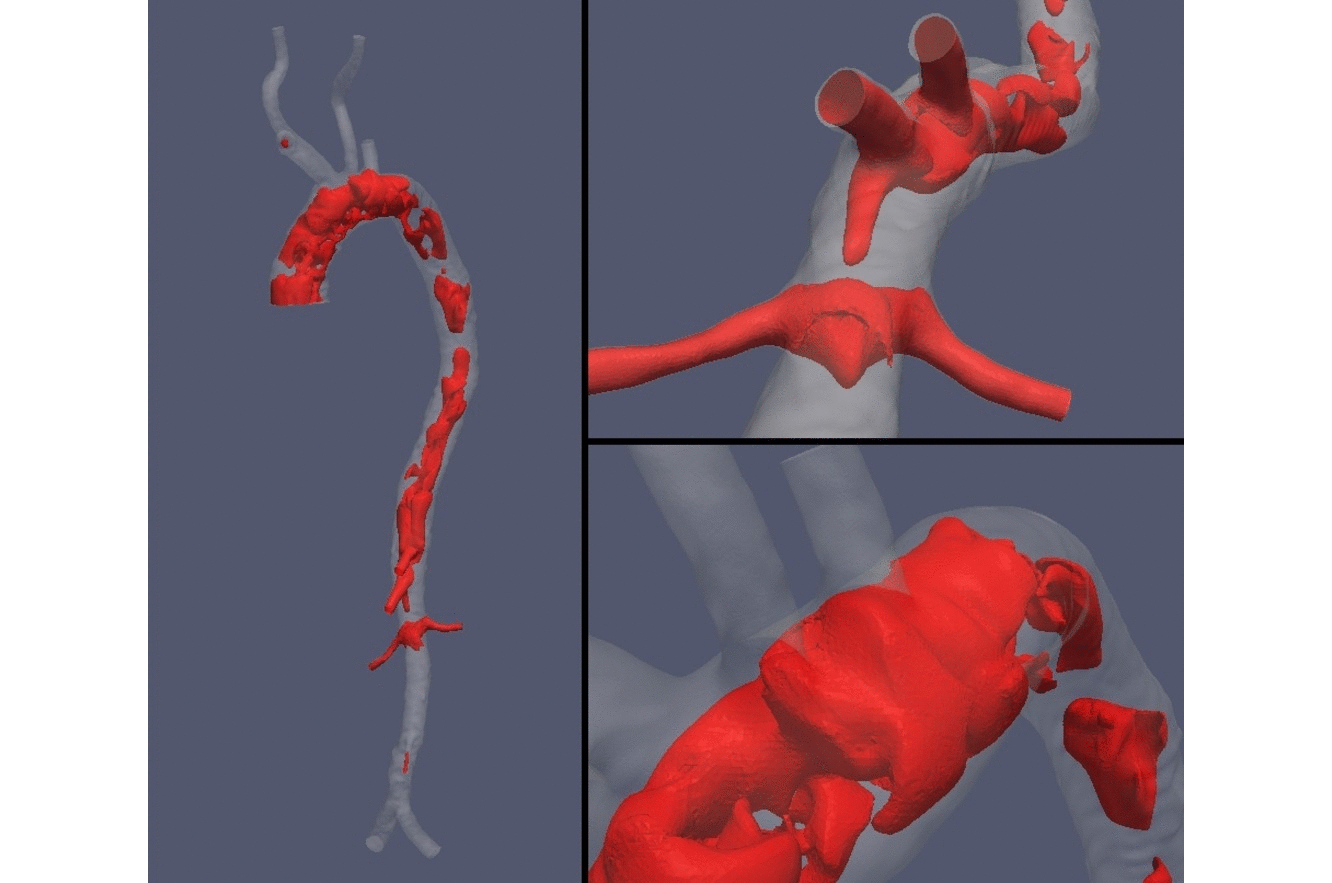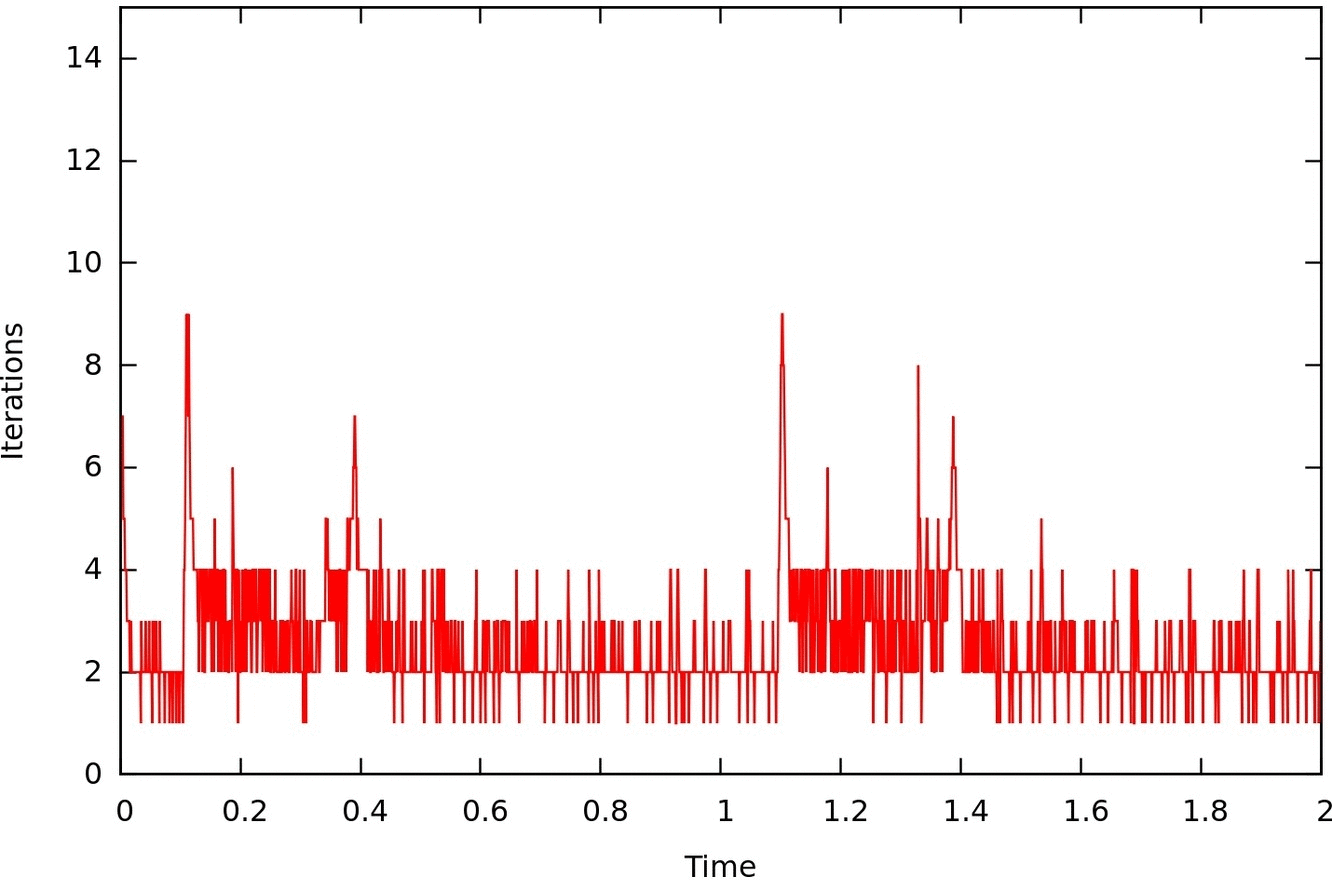
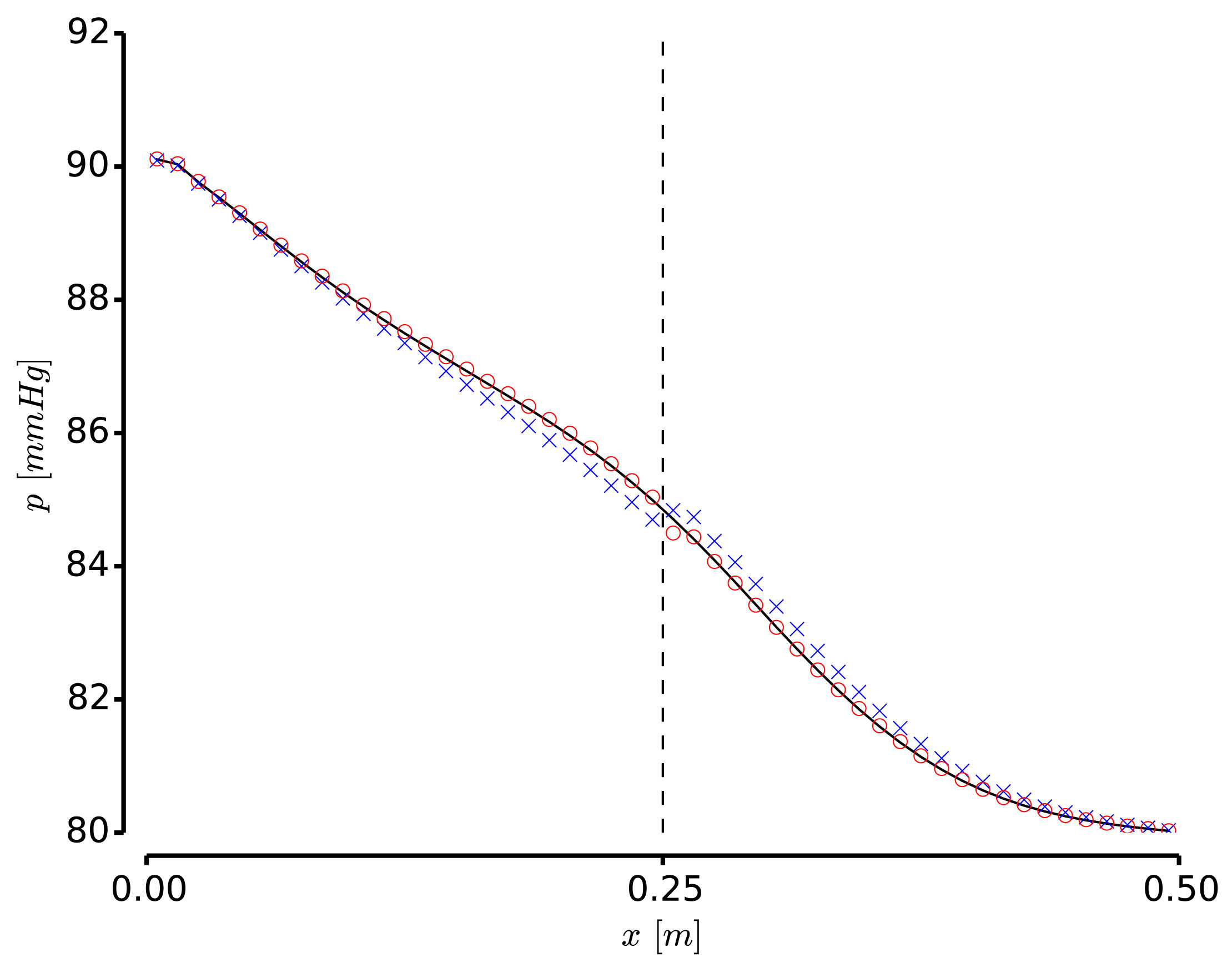
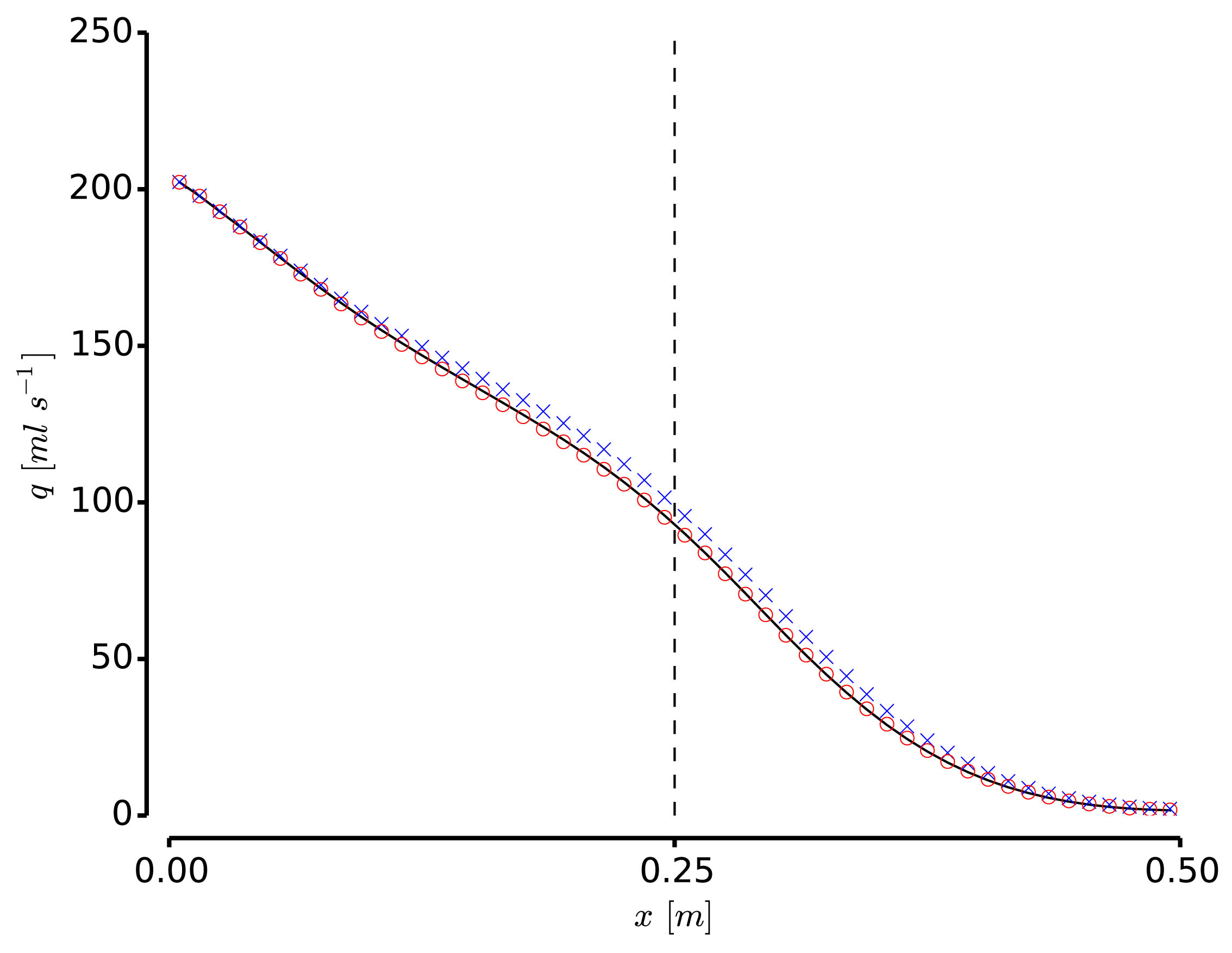
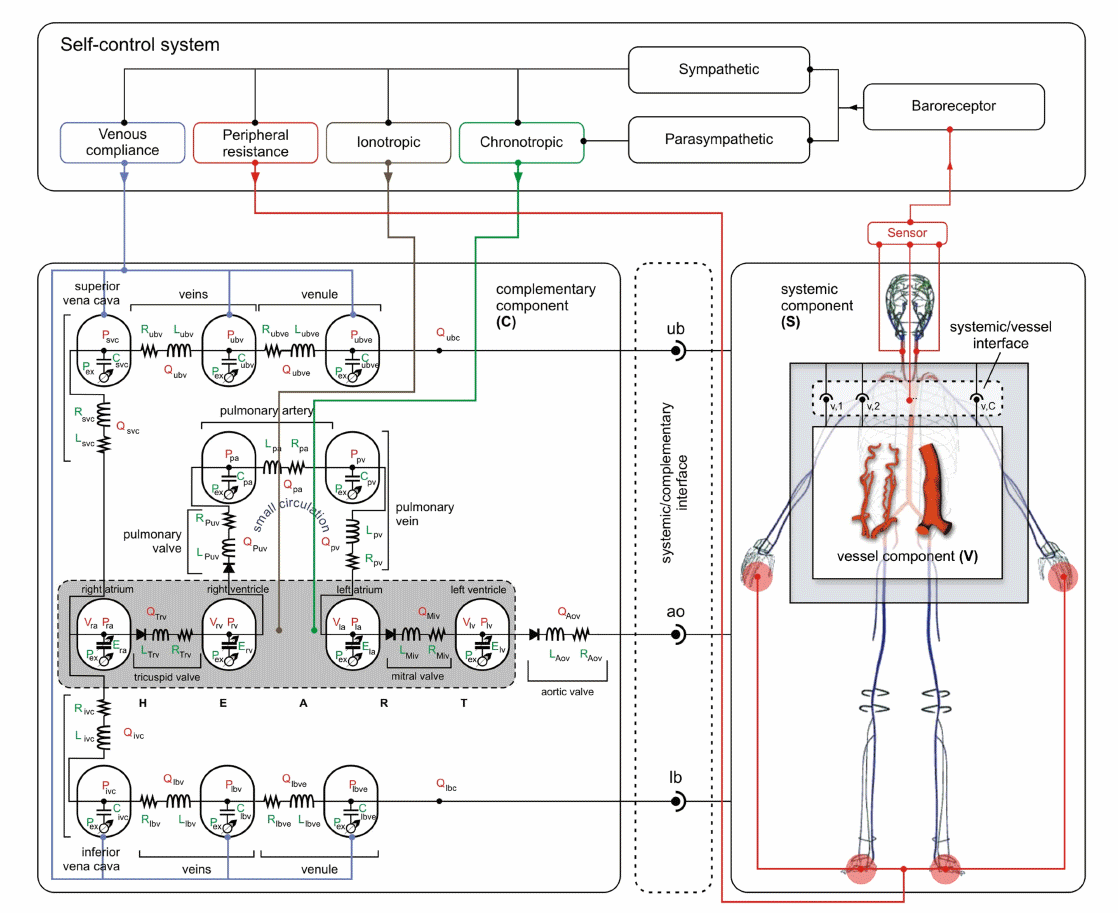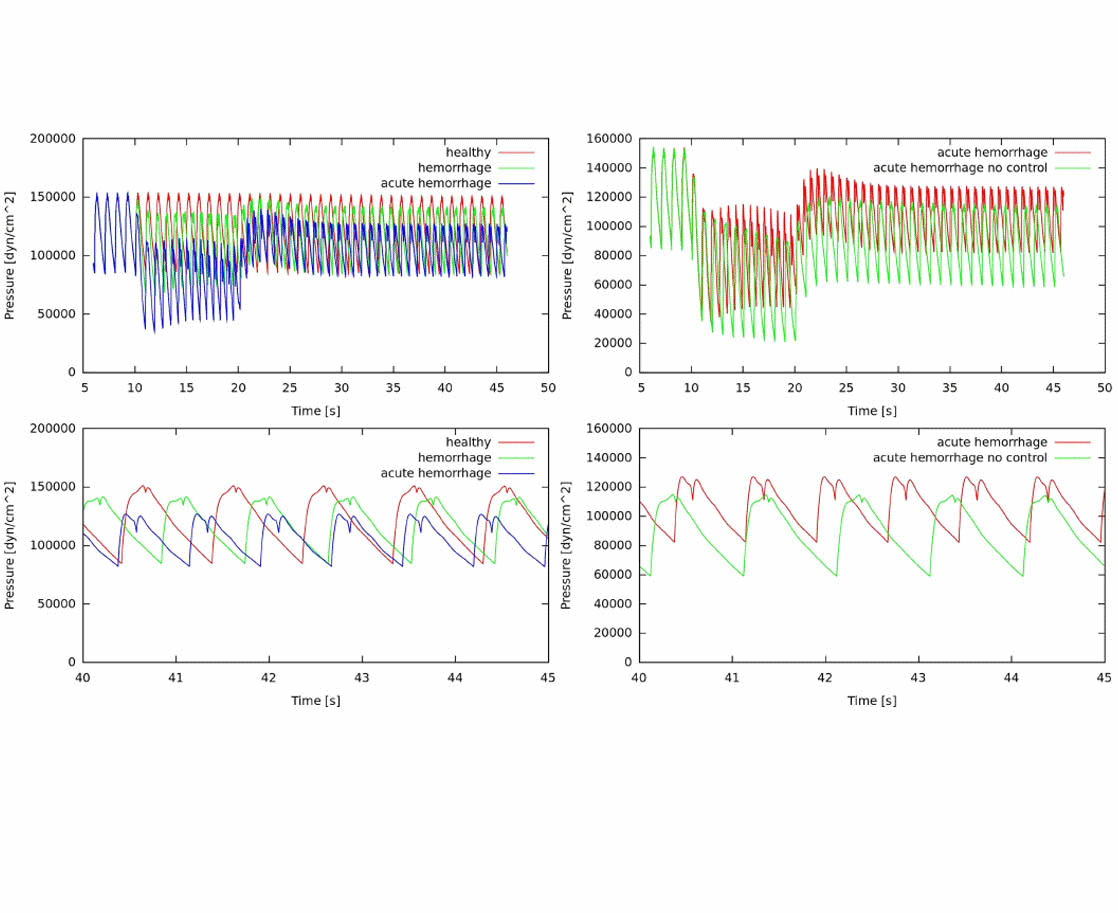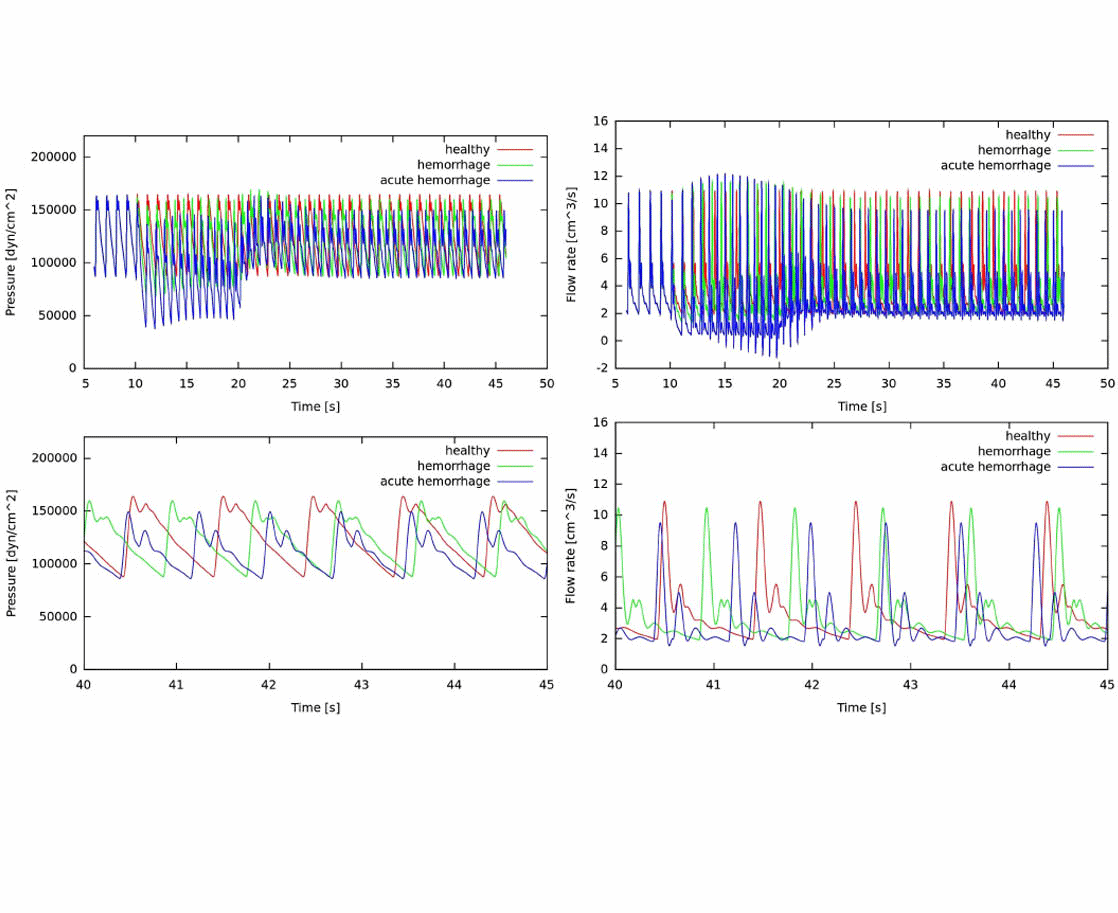
The development of anatomically accurate and highly detailed networks of vessels to perform 1D blood flow simulations involving wave propagation phenomena is of the utmost importance for the correct assessment of coarse and fine hemodynamics interactions throughout the entire cardiovascular system. In fact, models of this kind can be effectively used as auxiliary tools to understand underlying mechanisms of certain cardiovascular diseases as well as in the diagnosis and surgical planning. The ADAN (Anatomically Detailed Arterial Network) model developed by the HeMoLab group is the largest arterial network currently available, comprising all the arterial vessels acknowledged in the specialized literature. For such network specific numerical methods have been devised. The ADAN model can be coupled to 3D blood flow models to account for local-global hemodynamic interactions. Currently, it is being extended to incorporate the venous network to model arterio-venous interactions, as well as to account for autoregulatory mechanisms and transport of species among other phenomena. |
|
| 3D Blood Flow FSI Simulation |
|
|
expand
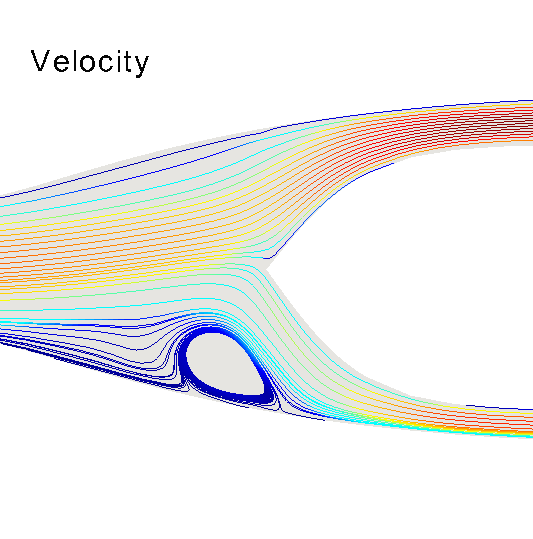
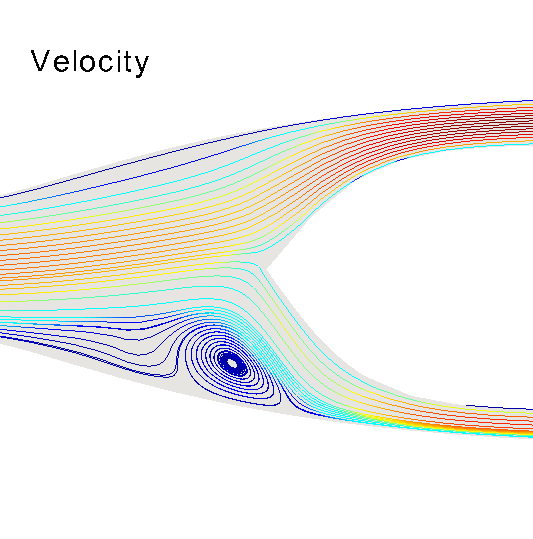
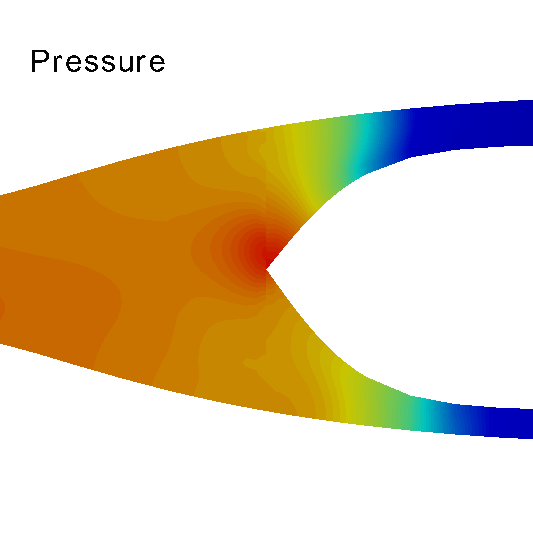
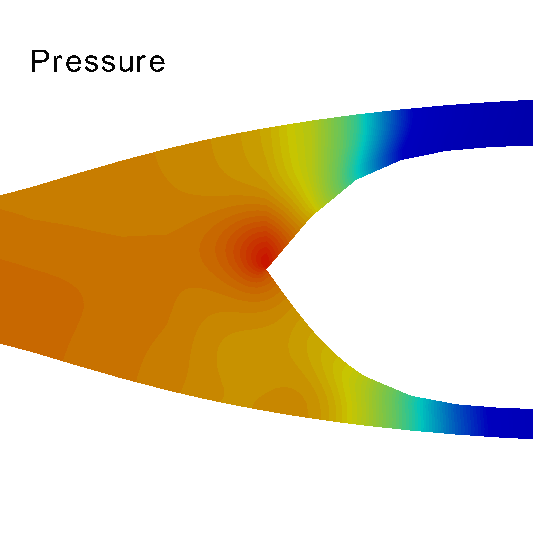
Three-dimensional hemodynamics simulations help to understand extremely fine mechanisms underlying blood-wall interactions, and the impact on flow disturbances, endothelial function and arterial wall damage, among other phenomena. Models of this kind can be applied to study the risk of rupture in the arterial wall, with implications in aneurysm rupture and thrombosis. In the HeMoLab group, we are concerned with performing realistic 3D fluid-structure simulations. This implies considering for the 3D models proper preload and prestress conditions of the arterial wall, blood wall interactions in the finite strain kinematical regime, proper flow boundary conditions through the coupling with 1D models, histologically-based constitutive equations and the effect of external tissues. Currently, we are interested in the integration of all these aspects to accurately assess the differential impact on the mechanical state and damage produced in the arterial walls, towards studying tissue rupture. |
|
| Numerical Methods for Dimensionally-Heterogeneous Models |
|
|
expand
Numerical methods to couple in an efficient way models of different dimensionality (for instance 1D and 3D blood flow models) are fundamental for the partitioned and parallel solution of coupled simulations. At the HeMoLab group we have developed specific iterative coupling methods to robustly and efficiently address this problem, allowing to solve in a fully parallel manner, for example, a 1D arterial network and the 3D models (with a further inner level of parallelism) embedded in it. |
|
| Numerical Methods for Hyperbolic Problems |
|
|
expand
Finite volume-based numerical methods can be employed in order to solve 1D blood flow equations in highly complex networks as well as eventual transport of species in the blood flow in an accurate, robust and computationally cheap manner. At the HeMoLab group, we have developed and implemented high order finite volume methods for the approximate solution of 1D blood flow, incorporating nonlinear terms introduced by collagen fibers and viscoelasticity of the arterial wall. In addition, high order methods for the treatment of junctions have also been developed for a consistent high order approach to the problem. These methods are fundamental tools for the numerical simulations involving the ADAN model. |
|
| Integrated Physiological Models (Download) |
|
|
expand
The cardiovascular system response is actually the result the response of several coupled systems acting as a functional physiological unit ensemble. From the interaction with the respiratory system to the subordination to autoregulatory systems, global and local hemodynamics conditions are highly dependent on the resulting homeostatic functional point. At the HeMoLab, we are interested in the coupling of blood circulation and gas transport in the system, as well as with short and mid term control mechanisms to account for most of the cardiovascular scenarios. A rational approach is pursued, by employing building blocks which can be substituted by even more complex mathematical representations, ultimately reaching an integrated anatomically and functionally detailed physiological model. |
|
| Automatic Vascularization Algorithms |
|
|
expand
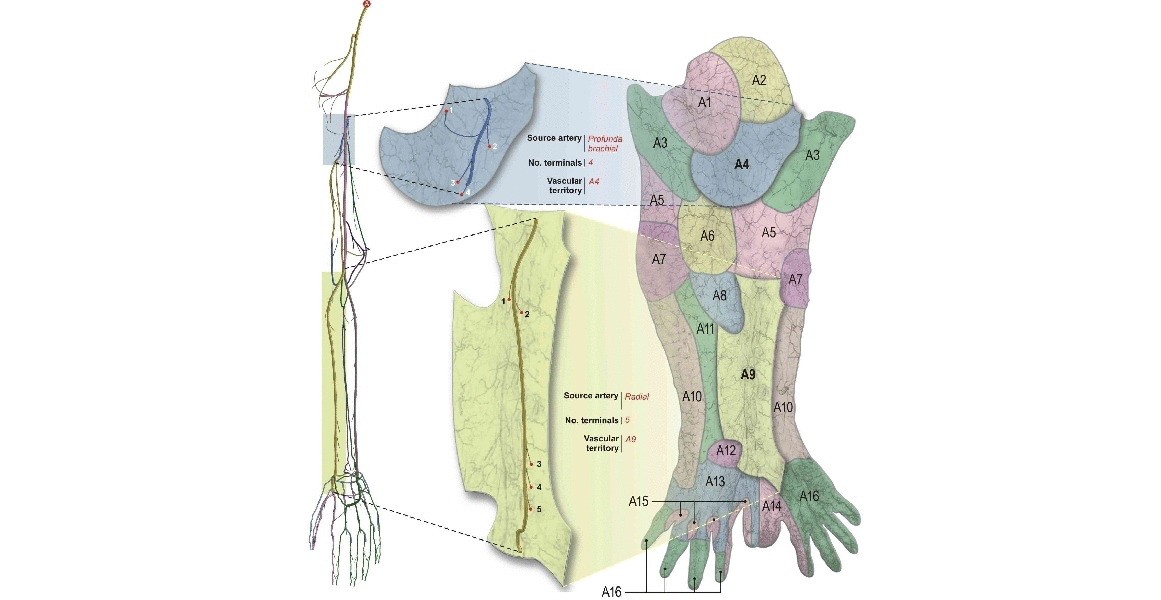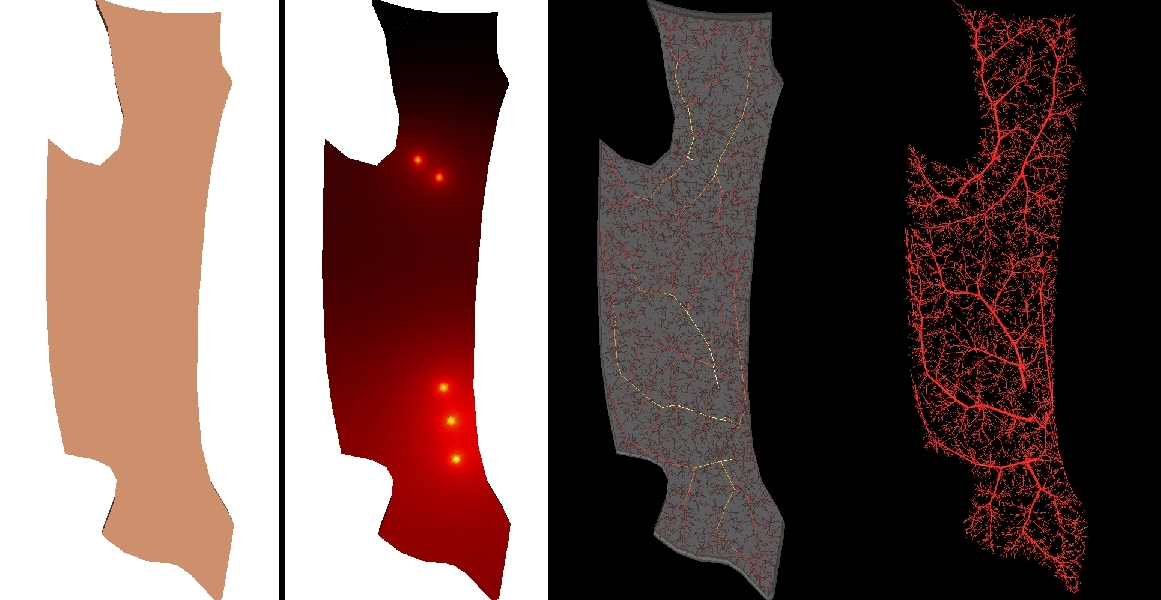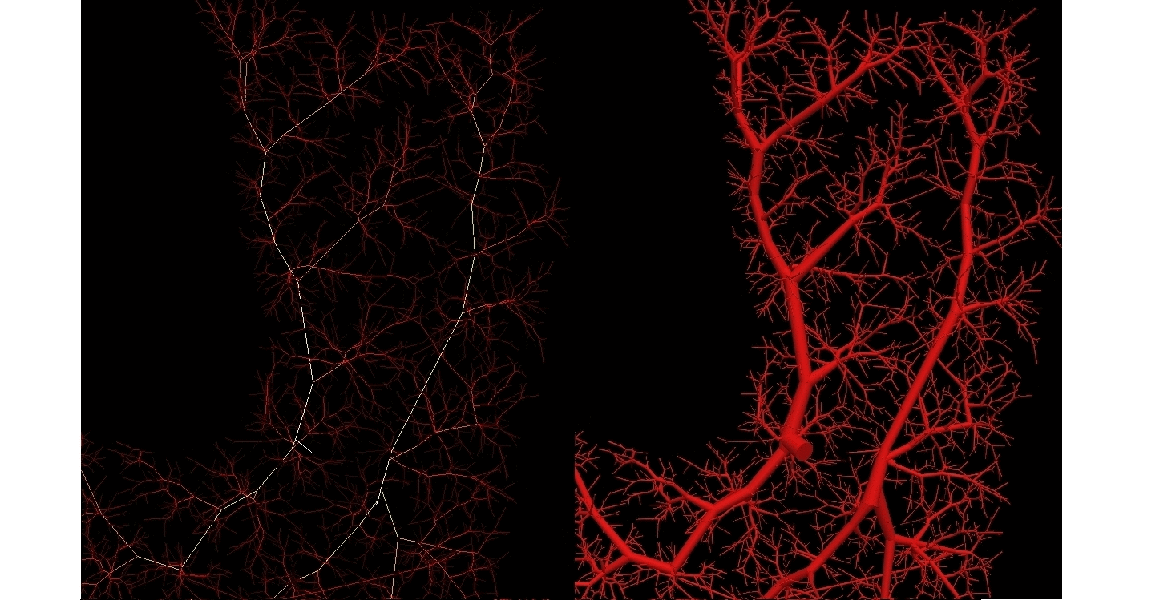
Arterial networks involving small vessels impose severe restrictions regarding the data acquisition and construction. To circumvent this, automatic generation of vascular networks is a strategy to create statistically meaningful models either to provide termination conditions in arterial vessels as well as to study in detail hemodynamic conditions in peripheral regions. At the HeMoLab, we have developed Constrained Constructive Optimization based algorithms to generate networks of vessels in vascular territories of arbitrary shape and with an arbitrary number of feeding arteries. |
|
| Computed Tomography Image Processing |
|
|
expand
Computed tomography medical image are fundamental datasets for the patient-specific characterization of anatomical structures of interest. In computational hemodynamics, proper processing and segmentation are of the utmost importance for the extraction of vessels surfaces and volumes towards the characterization of geometrical indexes and the subsequent use in blood flow simulations. At the HeMoLab we are concerned with robust methods based on level-sets methods for the filtering and segmentation of coronary and cerebral computed tomography images to feed patient-specific models to perform blood flow simulations and assess risk of miocardial infarction and risk of aneurysm rupture, respectively. |
|
| Intravascular Ultrasound Image Processing |
|
|
expand
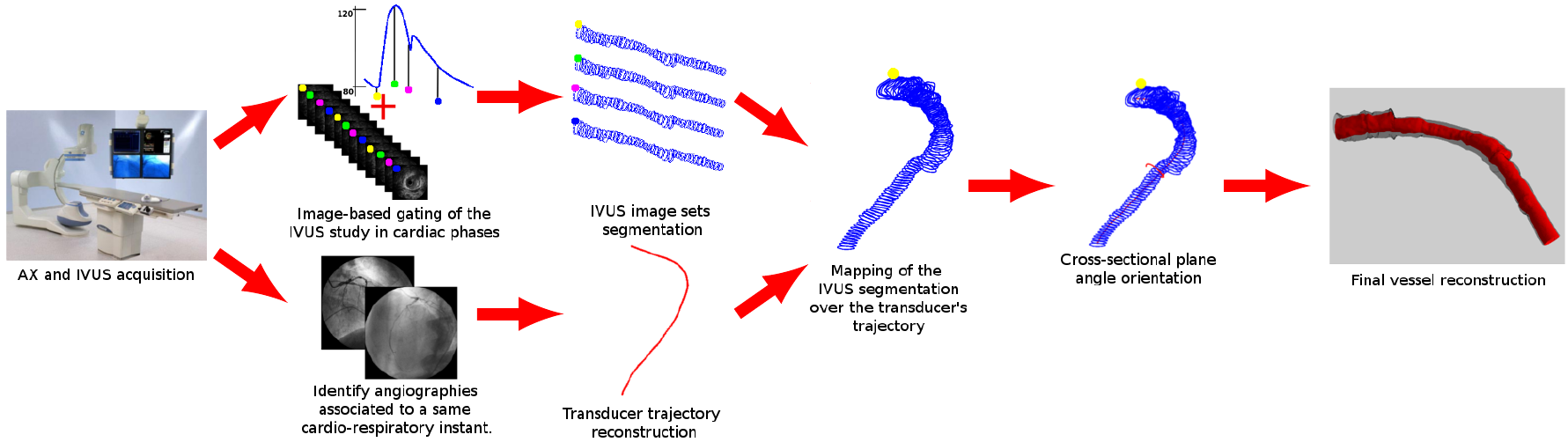
Intravascular ultrasound images are the gold standard for the image-based assessment of coronary diseases due to the large amount of data and high resolution provided for the transversal sections of coronary vessels. These images feature several artifact from the ultrasound nature as well as from external stimuli (respiration, cardiac motion, catheter motion, etc.). From image gating, going through image registration and segmentation, and finally wall motion quantification, at the HeMoLab we are interested in the entire pipeline to reach, from the medical dataset, by combination with angiographic images, accurate patient-specific physical models involving fluid and solid mechanics, as the bases to develop algorithms for in vivo tissue characterization. |
|
| Fast Hybrid Solvers in Blood Flow Simulations |
|
|
expand
There is increasing need for fast blood flow simulations. This need is supplied by approximation strategies, through the creation of problem specific basis functions, which take into account the very nature of the problem. This would allow practitioners to perform simulations in a timely manner while maintaining accuracy in wall shear stress and pressure drop predictions. At the HeMoLab, we are concerned with the development of spectral/finite element hybrid approximations for flow simulations taking advantage of the pipe-like domains as an in-between approach between expensive traditional 3D models and cheap, but incomplete, 1D models. |
|
| RVE-based Multiscale Modeling of Tissues |
|
|
expand
The constitutive behavior of complex biological tissues is quite complex in terms of the micro-mechanical interactions that ultimately give rise to a certain macroscopic observable response. A rational approach to address this problem is through the use of constitutive multiscale models. At the HeMoLab, we have developed a variational framework based on the principle of virtual power for the development of complex multiscale models. This framework is being currently used for the multiscale mechanical analysis of soft tissues, with special attention in the mechanisms behind tissue rupture. |
|